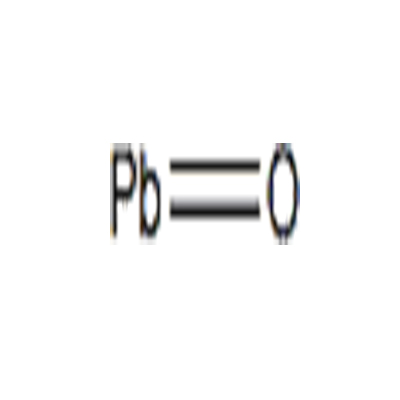Lead monoxide CAS#1317-36-8
CAS Number: 1317-36-8
Chemical Formula: OPb
Synonyms:
yellow lead monoxide
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Appearance: Yellow Powder
Lead monoxide is a yellow tetragonal powder; insoluble in water and ethanol; soluble in acetone, nitric acid, caustic soda and ammonium chloride.
It is amphoteric oxide and thus dissolves both in acids and in alkalies.
PbO + 2HNO3-->Pb(NO3)2+ H2O
PbO+ 2NaOH -->Na2PbO2(Sod. plumbite)+ H2O
On heating in air at 470°C it changes into red lead.
6PbO +O2-->2Pb3O4(red lead)
Lead monoxide Chemical Properties |
Melting point | 886 °C(lit.) |
Boiling point | 1470 °C |
bulk density | 3500-3700kg/m3 |
density | 9.53 |
vapor pressure | 10 mm Hg ( 0 °C) |
refractive index | 2.67 |
storage temp. | Store below +30°C. |
solubility | Soluble in concentrated alkali, HCl and ammonium chloride. Insoluble in dilute alkali and alcohol. |
form | powder |
color | yellow |
Specific Gravity | 9.53 |
PH | 8-9 (100g/l, H2O, 20℃)(slurry) |
Water Solubility | Soluble in concentrated alkali, hydrochloric acid, and ammonium chloride. Insoluble in water, dilute alkali and alcohol. |
Crystal Structure | Rutile type |
Hydrolytic Sensitivity | 4: no reaction with water under neutral conditions |
crystal system | square |
Merck | 14,5413 |
Space group | P42/mnm |
Lattice constant | a/nmb/nmc/nmα/oβ/oγ/oV/nm30.49550.49550.33839090900.0831 |
Exposure limits | ACGIH: TWA 0.05 mg/m3 |
Stability: | Stable. Reacts violently with hydrogen peroxide, strong oxidizing agents, aluminium, zirconium, halogens, sulphur trioxide, boron, silicon, sodium, zinc. |
InChI | 1S/O.Pb |
InChIKey | YEXPOXQUZXUXJW-UHFFFAOYSA-N |
SMILES | O=[PbH2] |
CAS DataBase Reference | 1317-36-8(CAS DataBase Reference) |
NIST Chemistry Reference | Lead monoxide(1317-36-8) |
EPA Substance Registry System | Lead monoxide (1317-36-8) |
Safety Information |
Hazard Codes | T,N |
Risk Statements | 61-20/22-33-50/53-62 |
Safety Statements | 53-45-60-61 |
RIDADR | UN 2291 6.1/PG 3 |
WGK Germany | 3 |
RTECS | OG1750000 |
TSCA | TSCA listed |
HS Code | 2824 10 00 |
HazardClass | 6.1(b) |
PackingGroup | III |
Storage Class | 6.1C - Combustible acute toxic Cat.3 |
Hazard Classifications | Acute Tox. 4 Inhalation |
Hazardous Substances Data | 1317-36-8(Hazardous Substances Data) |
Toxicity | LD50 i.p. in rats: 40 mg Pb/100g (Bradley, Fredrick) |
Product Application of Lead monoxide CAS#1317-36-8
Lead(II) Oxide is used as the starting material in the synthesis of Dibasic Lead Phthalate Hydrate (D417070); a compound that is used in vinyl wire insulation as a flame retardant.
The principal use of lead monoxide is in the manufacture of pastes for the grids used in lead-acid batteries. It is also widely used in optical, electrical, and electronic glasses, as well as in glazes for fine tableware.To render the lead compounds insoluble in foods, the glazes and vitreous enamels are prepared from frits, in which the lead monoxide is converted to lead bisilicate, for example.
Litharge is also used in rubber as a vulcanizing agent, in lead soaps employed as driers in varnishes, in high-temperature lubricants, as a neutralizing agent in organic syntheses, as a heat stabilizer in plastics, and as a starting material in the production of pigments. The preparation, properties, and other uses of lead oxide are described in.
Fact Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days