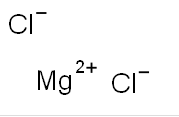Magnesium chlorideCAS#7786-30-3
Chemical Name:Magnesium chloride
CAS No.:7786-30-3
Appearance: colorless powder
Molecular Formula:Cl2Mg
Molecular weight:95.21
Sample: Available
Products Description of Magnesium chlorideCAS#7786-30-3
Magnesium chloride (anhydrous) industrial product purity ≥ 99%, white crystalline powder, odorless, highly hygroscopic. Melting point 714℃, boiling point 1412℃. Vapor pressure negligible (25℃), relative density 2.32 (20℃). Solubility in water: highly soluble, soluble in ethanol, insoluble in ether. Stable, incompatible with strong oxidants and strong bases.
Parameters
Melting point | 714 °C (lit.) |
Boiling point | 1412 °C/1 atm (lit.) |
density | 2.32 g/mL at 25 °C (lit.) |
refractive index | n20/D 1.336 |
storage temp. | 2-8°C |
solubility | H2O: soluble |
form | powder |
color | colorless |
Specific Gravity | 2.41 |
Odor | odorless |
Flame Color | Colorless |
PH | 5.0-7.5 (25℃, 1M in H2O) |
Water Solubility | 400 G/L (20 ºC) |
λmax | λ: 260 nm Amax: 0.05 |
Sensitive | Hygroscopic |
Crystal Structure | CdCl2 type |
crystal system | Three sides |
Merck | 14,5662 |
Space group | R3m |
Lattice constant | a/nmb/nmc/nmα/oβ/oγ/oV/nm30.3640.3641.76790901200.2023 |
Stability: | hygroscopic |
Cosmetics Ingredients Functions | FLAVOURING |
CAS DataBase Reference | 7786-30-3(CAS DataBase Reference) |
NIST Chemistry Reference | Magnesium dichloride(7786-30-3) |
EPA Substance Registry System | Magnesium chloride (7786-30-3) |
Safety Information |
Hazard Codes | Xi |
Risk Statements | 36/37/38-43-36/37 |
Safety Statements | 26-36-24/25-22-36/37-39 |
WGK Germany | 1 |
RTECS | OM2800000 |
F | 3-10 |
TSCA | Yes |
HS Code | 28273100 |
Hazardous Substances Data | 7786-30-3(Hazardous Substances Data) |
Toxicity | LD50 orally in Rabbit: 2800 mg/kg |
Product Application of Magnesium chlorideCAS#7786-30-3
Magnesium chloride is used for a variety of other applications besides the production of Mg metal. It is used in the manufacture of textiles, paper, fireproofing agents, cements and refrigeration brine. It has also been used in dust and erosion control. Mixed with hydrated magnesium oxide, magnesium chloride forms a hard material called “Sorel-cement”. The cement is a mixture of MgO (burnt magnesia) with MgCl2 with the approximate chemical formula Mg4Cl2(OH)6(H2O)8, corresponding to a weight ratio of 2.5–3.5 parts MgO to one part MgCl2. This usage of magnesium chloride is unmatched by any other compound. MgCl2 was one of the earlier antiseptics, first used for that purpose by Dr Pierre Delbert in 1915. One veterinary study in 1989 indicated some effectiveness against tumors when used as a feed additive.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days