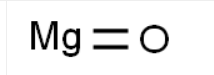Magnesium oxide CAS#1309-48-4
CAS Number: 1309-48-4
Chemical Formula: MgO
Synonyms:
slo469;Tanbase
Appearance: White power
HS Code: 2519909100
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Products Description of Magnesium oxide CAS#1309-48-4
Magnesium oxide is commonly known as magnesia, also known as magnesium oxide. It is a typical alkaline earth metal oxide with the chemical formula MgO. It is a white powder with a melting point of 2852℃, a boiling point of 3600℃ and a relative density of 3.58 (25℃). It is soluble in acid and ammonium salt solutions. It reacts slowly with water to form magnesium hydroxide. It can be dissolved in a carbon dioxide aqueous solution to form magnesium bicarbonate. It can gradually absorb water and carbon dioxide in the air, and emit irritating smoke when heated. Magnesite (MgCO3), dolomite (MgCO3·CaCO3) and seawater are the main raw materials for the production of magnesium oxide. Magnesium oxide is obtained by thermal decomposition of magnesia or dolomite. Magnesium hydroxide precipitate is obtained by treating seawater with slaked lime, and magnesium hydroxide is burned to obtain magnesium oxide. Magnesium chloride brine blocks obtained from the comprehensive utilization of seawater or brine after bromine extraction can also be used as raw materials, sodium hydroxide or sodium carbonate are added to generate magnesium hydroxide or basic magnesium carbonate precipitate, and then burned to obtain magnesium oxide. At present, China mainly uses magnesia, dolomite, brine or brine blocks as raw materials. Magnesium oxide has the largest output among magnesium compounds, accounting for about 3/4 of all magnesium industrial products Chemicalbook. Magnesium oxide produced below 900℃ is light magnesium oxide, with low density, large specific surface area and strong adsorption. It can be used as a catalyst, rubber filler and accelerator for improving rubber properties. It is mixed with magnesium chloride solution to make magnesium cement. It is used as a flame retardant for building materials. It is used as an antacid and laxative in medicine for hyperacidity and gastric and duodenal ulcers, and is often used in combination with calcium carbonate that is prone to constipation. It is used as an animal feed additive and plant fertilizer. Light magnesium oxide produced at 950-1050℃ has a large density, a certain range of particle distribution, and is easier to combine with water. It can be used as an isolation agent for silicon steel plates to prevent sintering and adhesion during high-temperature treatment by reacting with silicon dioxide on the surface of silicon steel at high temperature to form a magnesium silicate film. Heavy magnesium oxide produced at 1500-1800℃ has high density, small specific surface area, is not easily decomposed by heat, has low chemical activity, is not easy to react with acid, and has low hydration rate. It is used as high-temperature refractory material, binder for making refractory crucibles and furnace linings, etc.
Parameters
| Melting point | 2852 °C (lit.) |
| Boiling point | 3600 °C |
| density | 3.58 |
| bulk density | 100kg/m3 |
| refractive index | 1.736 |
| Fp | 3600°C |
| storage temp. | no restrictions. |
| solubility | 5 M HCl: 0.1 M at 20 °C, clear, colorless |
| form | nanopowder |
| color | White |
| Specific Gravity | 3.58 |
| Odor | wh. powd. or cryst., odorless |
| PH | 10.3 (H2O, 20℃)(saturated solution) |
| Resistivity | 1.3 ∞ 10*15 (ρ/μΩ.cm) |
| Water Solubility | 6.2 mg/L (20 ºC), reacts |
| semiconductor properties | <111> |
| Crystal Structure | Cubic |
| Sensitive | Air Sensitive |
| λmax | λ: 260 nm Amax: ≤0.040 λ: 280 nm Amax: ≤0.025 |
| Merck | 14,5677 |
| crystal system | Cube |
| Space group | Fm3m |
| Lattice constant | a/nmb/nmc/nmα/oβ/oγ/oV/nm30.42270.42270.42279090900.0755 |
| Dielectric constant | 9.7(0.0℃) |
| Exposure limits | ACGIH: TWA 10 mg/m3 OSHA: TWA 15 mg/m3 NIOSH: IDLH 750 mg/m3 |
| Stability: | Stable. Incompatible with bromine trifluoride, bromine trichloride, phosphorus pentachloride. |
| InChIKey | CPLXHLVBOLITMK-UHFFFAOYSA-N |
| CAS DataBase Reference | 1309-48-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Magnesium monoxide(1309-48-4) |
| EPA Substance Registry System | Magnesium oxide (1309-48-4) |
Safety Information
| HS Code | 25199099 |
| Hazardous Substances Data | 1309-48-4(Hazardous Substances Data) |
| Toxicity | TCLo inhalation in human: 400mg/m3 |
| IDLA | 750 mg/m3 |
Products Description of Magnesium oxide CAS#1309-48-4
Inorganic chemical products; Inorganic salts; Common analytical reagents; Standard products; Biochemical reagents; Cj magnesium compounds; Light metals; Catalysis and inorganic chemistry; Gastrointestinal drugs; APIs; Food additives; Pharmaceutical excipients; Biochemical reagents-other chemical reagents; Nutritional enhancers; Organic chemical raw materials; Coating materials; Metal oxides; Inorganic chemistry; General reagents; Inorganic nanomaterials; Magnesium; Oxides; Inorganic compounds and salts; Solvents; Organic chemistry; Pharmaceutical raw materials; Reagents for specific purposes; Chemical products-Organic solvents; Nutritional enhancers; Conventional oxide powder-Magnesium oxide; Chemical raw materials; Chemical products; Raw materials; Oxides-Magnesium oxide; Inorganic acids
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days