Sodium Bicarbonate CAS#144-55-8
CAS Number: 144-55-8
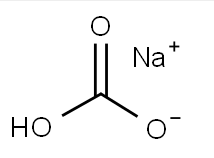
Chemical Formula: CHNaO3
Synonyms:
TSQN
Sodium bicarbonate Manufacturer
Sodium bicarbonate test solution(ChP
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Appearance: Colorless transperant liquid
Sodium Bicarbonate CAS#144-55-8
Sodium bicarbonate, which is the compound commonly called baking soda, exists as a white, odorless, crystalline solid. It occurs naturally as the mineral nahcolite, which derives its name from its chemical formula by replacing the “3” in NaHCO3 with the ending “lite.” The world’s main source of nahcolite is the Piceance Creek Basin in western Colorado, which is part of the larger Green River formation. Sodium bicarbonate is extracted using solution mining by pumping hot water through injection wells to dissolve the nahcolite from the Eocene beds where it occurs 1,500 to 2,000 feet below the surface. The dissolved sodium bicarbonate is pumped to the surface where it is treated to recover NaHCO3 from solution. Sodium bicarbonate can also be produced from the trona deposits, which is a source of sodium carbonates (see Sodium Carbonate).
| Sodium bicarbonate Chemical Properties |
| Melting point | >300 °C(lit.) |
| Boiling point | 851°C |
| bulk density | 1000kg/m3 |
| density | 2.16 g/mL at 25 °C (lit.) |
| refractive index | 1.500 |
| storage temp. | 2-8°C |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | solution (7.5%) |
| color | White |
| Specific Gravity | 2.159 |
| Odor | Odorless |
| PH | 8.27(1 mM solution);8.22(10 mM solution);8.02(100 mM solution); |
| PH Range | 7.8 - 8.2 |
| pka | (1) 6.37, (2) 10.25 (carbonic (at 25℃) |
| Water Solubility | 9 g/100 mL (20 ºC) |
| Decomposition | 50 °C |
| Merck | 14,8583 |
| BRN | 4153970 |
| BCS Class | 1 |
| Stability: | Stable. |
| Cosmetics Ingredients Functions | DEODORANT BUFFERING ABRASIVE ORAL CARE SKIN PROTECTING |
| LogP | -4.010 (est) |
| CAS DataBase Reference | 144-55-8(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium bicarbonate (144-55-8) |
| Safety Information |
| Safety Statements | 24/25 |
| WGK Germany | 1 |
| RTECS | VZ0950000 |
| TSCA | Yes |
| HS Code | 28363000 |
| Hazardous Substances Data | 144-55-8(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 4220 mg/kg |
Product Application of Sodium Bicarbonate CAS#144-55-8
Sodium bicarbonate, used in the formof baking soda and baking powder, is the most common leavening agent. When baking soda,which is an alkaline substance, is added to a mix, it reacts with an acid ingredient to producecarbon dioxide. The reaction can be represented as: NaHCO3(s) + H+ → Na+(aq) + H2O(l) +CO2(g), where H+ is supplied by the acid. Baking powders contain baking soda as a primaryingredient along with acid and other ingredients. Depending on the formulation, bakingpowders can produce carbon dioxide quickly as a single action powder or in stages, as with adouble-action powder. Baking soda is also used as a source of carbon dioxide for carbonatedbeverages and as a buffer.In addition to baking, baking soda has numerous household uses. It is used as a generalcleanser, a deodorizer, an antacid, a fire suppressant, and in personal products such as toothpaste.Sodium bicarbonate is a weak base in aqueous solution, with a pH of about 8. Thebicarbonate ion (HCO3-) has amphoteric properties, which means it can act as either an acidor a base. This gives baking soda a buff ering capacity and the ability to neutralize both acidsand bases. Food odors resulting from acidic or basic compounds can be neutralized with bakingsoda into odor-free salts. Because sodium bicarbonate is a weak base, it has a greater abilityto neutralize acid odors.
The second largest use of sodium bicarbonate, accounting for approximately 25% of totalproduction, is as an agricultural feed supplement. In cattle it helps maintain rumen pH andaids fiber digestibility; for poultry it helps maintain electrolyte balance by providing sodiumin the diet, helps fowl tolerate heat, and improves eggshell quality.
Sodium bicarbonate is used in the chemical industry as a buff ering agent, a blowingagent, a catalyst, and a chemical feedstock. Sodium bicarbonate is used in the leather tanningindustry for pretreating and cleaning hides and to control pH during the tanning process.Heating sodium bicarbonate produces sodium carbonate, which is used for soap and glassmaking.Sodium bicarbonate is incorporated into pharmaceuticals to serve as an antacid, abuff ering agent, and in formulations as a source of carbon dioxide in eff ervescent tablets. Drychemical type BC fire extinguishers contain sodium bicarbonate (or potassium bicarbonate).Other uses of bicarbonate include pulp and paper processing, water treatment, and oil welldrilling.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days