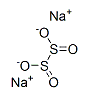
Sodium dithionite CAS#7775-14-6
CAS Number: 7775-14-6
Chemical Formula: Na2O4S2
Synonyms:
disodiumdithionite
SODIUM HYDROSULPHITE
dithionous acid disodium salt
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Appearance: White Powder
Sodium dithionite CAS#7775-14-6
Sodium dithionite is also called sodium hydrosulfite, sodium hydrosulphite, sodium sulfoxylate, and sulfoxylate. Sodium dithionite is not stable under physiological conditions, with the rate of decomposition increasing with increasing acidity. Upon contact with moisture, it is oxidized to hydrogen sulfite (HSO3-), sulfite (SO32-) and hydrogen sulfate (HSO4-). Under strongly acidic conditions it may liberate sulfur dioxide. Under anaerobic conditions (such as in the lower gastrointestinal tract), hydrogen sulfite (HSO3-) and thiosulfate (S2O32-) may be formed. Hydrogen sulfite (HSO3-) can be absorbed after ingestion. It is efficiently metabolized and the major part rapidly is excreted as sulfate into the urine.
Sodium dithionite is widely used in industry owing to its reducing properties and ability to react with oxygen. It is used in textile industry for dyeing, in the pulp and paper industry as a reducing bleach to remove yellow discoloration from cellulose based products, as an oxygen scavenger in boilers, in conservation to remove iron stains on cultural artifacts, and in water treatment for controlling iron flash on white fabrics in bleaching environments. It is also used in photographic film, clay, wine, leather goods, foods and beverages, polymers, cleaners, gas purification, environmental remediation, metal recovery, and chemical processing.
Sodium dithionite Chemical Properties |
Melting point | 300 °C |
Boiling point | 1390°C |
bulk density | 1250kg/m3 |
density | 2.13 |
Fp | >100°C |
storage temp. | Store at +5°C to +30°C. |
solubility | 250 g/L (20°C) |
form | Powder/Solid |
color | White |
Odor | None or slight scent of sulfur dioxide |
PH | 5.5-8.5 (50g/l, H2O, 20℃) |
Water Solubility | 250 g/L (20 ºC) |
Sensitive | Moisture Sensitive |
Merck | 14,8626 |
Stability: | Stable, but air sensitive. Incompatible with strong acids, strong oxidizing agents, water, moisture. |
Cosmetics Ingredients Functions | VISCOSITY CONTROLLING |
InChI | 1S/2Na.H2O4S2/c;;1-5(2)6(3)4/h;;(H,1,2)(H,3,4)/q2*+1;/p-2 |
InChIKey | JVBXVOWTABLYPX-UHFFFAOYSA-L |
SMILES | [Na+].[Na+].[O-]S(=O)S([O-])=O |
LogP | -2.756 (est) |
CAS DataBase Reference | 7775-14-6(CAS DataBase Reference) |
EPA Substance Registry System | Sodium hydrosulfite (7775-14-6) |
Safety Information |
Hazard Codes | Xn |
Risk Statements | 7-22-31 |
Safety Statements | 26-28-43-7/8-43E-28A |
RIDADR | UN 1384 4.2/PG 2 |
WGK Germany | 1 |
F | 1-10 |
Autoignition Temperature | >200 °C |
TSCA | TSCA listed |
HazardClass | 4.2 |
PackingGroup | II |
HS Code | 28311010 |
Storage Class | 4.2 - Pyrophoric and self-heating hazardous materials |
Hazard Classifications | Acute Tox. 4 Oral |
Hazardous Substances Data | 7775-14-6(Hazardous Substances Data) |
Toxicity | LD50 orally in Rabbit: 2500 mg/kg |
Product Application of Sodium dithionite CAS#7775-14-6
Sodium hydrosulfite is used as a reducing agent in aqueous solutions, sulfonating agent , chelating agent and decolorizing agent in organic reactions. It finds application in water treatment, gas purification, cleaning, leather, polymers, photography, and many others. It is involved in chemical enhanced oil recovery to stabilize polyacrylamide polymers against radical degradation in the presence of iron. It plays an important role to determine the iron content in soil chemistry.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days