Ethylenediaminetetraacetic acid CAS#60-00-4
CAS Number: 60-00-4
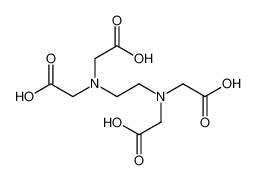
Chemical Formula: C10H16N2O8
Synonyms:
([2-(Bis-carboxymethyl-amino)-ethyl]-carboxymethyl-amino)-acetic acid
(ethylenedinitrilo)tetra-aceticaci
(Ethylenedintrilo)tetraacetic acid
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Appearance: Colorless Liquid
Ethylenediaminetetraacetic acid CAS#60-00-4
Ethylenediaminetetraacetic Acid (EDTA) is a common polydentate ligand. In EDTA, the hydrogen atoms are easily removed in solution to produce anionic EDTA4-. In its anionic form Ethylenediaminetetraacetic Acid (EDTA) has six binding atoms, two nitrogen and four oxygen. |
Ethylenediaminetetraacetic acid Chemical Properties |
Melting point | 250 °C (dec.) (lit.) |
Boiling point | 434.18°C (rough estimate) |
bulk density | 600kg/m3 |
density | 1.46 g/cm3 at 20 °C |
vapor pressure | <0.013 hPa (20 °C) |
refractive index | n |
Fp | >400°C DIN 51758 |
storage temp. | 2-8°C |
solubility | 3 M NaOH: 100 mg/mL |
form | crystalline |
pka | pKa 2 (Uncertain);10.26 (Uncertain) |
color | White to almost white |
Odor | Odorless |
PH | 2.5 (10g/l, H2O, 23℃)(slurry) |
PH Range | 2.5 at 10 g/l at 23 °C |
Water Solubility | 0.5 g/L (25 ºC) |
λmax | λ: 280 nm Amax: ≤0.25 |
Decomposition | 240 °C |
Merck | 14,3517 |
BRN | 1716295 |
Stability: | Stable. Incompatible with copper, copper alloys, nickel, aluminium, strong oxidizing agents, strong bases |
Cosmetics Ingredients Functions | CHELATING |
Cosmetic Ingredient Review (CIR) | Ethylenediaminetetraacetic acid (60-00-4) |
InChI | 1S/C10H16N2O8/c13-7(14)3-11(4-8(15)16)1-2-12(5-9(17)18)6-10(19)20/h1-6H2,(H,13,14)(H,15,16)(H,17,18)(H,19,20) |
InChIKey | KCXVZYZYPLLWCC-UHFFFAOYSA-N |
SMILES | OC(=O)CN(CCN(CC(O)=O)CC(O)=O)CC(O)=O |
LogP | -0.836 (est) |
CAS DataBase Reference | 60-00-4(CAS DataBase Reference) |
NIST Chemistry Reference | N,N'-1,2-Ethane diylbis-(N-(carboxymethyl)glycine)(60-00-4) |
EPA Substance Registry System | Ethylenediaminetetraacetic acid (60-00-4) |
Safety Information |
Hazard Codes | Xi |
Risk Statements | 36-52/53-36/37/38-36/38 |
Safety Statements | 26-61-37/39-36 |
RIDADR | UN 3077 9 / PGIII |
WGK Germany | 2 |
RTECS | AH4025000 |
F | 3 |
Autoignition Temperature | >200 °C |
TSCA | TSCA listed |
HS Code | 2922 49 85 |
HazardClass | 9 |
Storage Class | 11 - Combustible Solids |
Hazard Classifications | Eye Irrit. 2 |
Hazardous Substances Data | 60-00-4(Hazardous Substances Data) |
Toxicity | LD50 orally in Rabbit: 2580 mg/kg |
Product Application Of Ethylenediaminetetraacetic acid CAS#60-00-4
Edetic acid and edetate salts are used in pharmaceutical formulations, cosmetics, and foods as chelating agents. They form stable water-soluble complexes (chelates) with alkaline earth and heavy metal ions. The chelated form has few of the properties of the free ion, and for this reason chelating agents are often described as ‘removing’ ions from solution; this process is also called sequestering. The stability of the metal–edetate complex depends on the metal ion involved and also on the pH. The calcium chelate is relatively weak and will preferentially chelate heavy metals, such as iron, copper, and lead, with the release of calcium ions. For this reason, edetate calcium disodium is used therapeutically in cases of lead poisoning.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days