Calcium phosphate monobasic CAS#10031-30-8
CAS Number: 10031-30-8
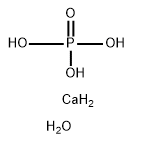
Chemical Formula: CaH7O5P
Synonyms:
PRIM-CALCIUM PHOSPHATE MONOHYDRATE
MONOCALCIUM PHOSPHATE
Calcium phosphate mo
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Appearance: White Powder
Calcium phosphate monobasic CAS#10031-30-8
Calcium phosphate monobasic monohydrate is mainly used in the manufacture of fertilizers and as an acidulant in food industry. Aqueous solution of calcium phosphate monobasic monohydrate (MCPM) mixed with α-tertiary calcium phosphate forms dicalcium phosphate dihydrate cement. It is also used in composing brushite cement.
| Calcium phosphate monobasic Chemical Properties |
| Melting point | 100°C |
| Boiling point | 203°C |
| density | 2.220^1^6 |
| Fp | 203°C |
| solubility | slightly soluble in H2O; soluble in dilute acid solutions |
| form | Powder |
| color | white |
| Odor | Odorless |
| Water Solubility | moderately soluble H2O; soluble dilute HCl, HNO3, acetic acid [MER06] |
| Merck | 14,1693 |
| Dielectric constant | 14.0(Ambient) |
| InChIKey | ZBZJARSYCHAEND-UHFFFAOYSA-L |
| CAS DataBase Reference | 10031-30-8(CAS DataBase Reference) |
| EPA Substance Registry System | Monocalcium phosphate monohydrate (10031-30-8) |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36/37 |
| WGK Germany | 2 |
| RTECS | TB8530000 |
| HS Code | 31031090 |
Product Application of Calcium phosphate monobasic CAS#10031-30-8
Monocalcium phosphate monohydrate was the first acidic phosphate used as a leavening acid. A patent for its use in baking powders was issued in 1956. The early MCP· H2 0 was prepared by ashing bones to form a crude product. This phosphate is classed as a fast-acting leavening acid; approximately 60% of the theoretical C02 is released by reaction of the MCP· H2 0 with soda during the mixing stage of batter or dough preparation. No further gas is then released until the product is placed in the oven and the temperature has reached approximately l40°F. The reason for this is that MCP· H2 0 disproportionates upon contact with water to form some dicalcium phosphate according to the following equation: Ca(H2PO4)2.H2O+H2O=CaHPO4.2H2O+H3PO4.
Because of the speed of its reaction, MCP· H20 has limited applications by itself. Although it is commonly used in the preparation of phosphated flour at levels of 0.25 to 0.75% of the weight of the finished product and in the preparation of cookies, most of its applications are in combination with other slower-acting phosphate leavening acids. It is expected to release gas during the mixing stages to assist in forming bubble nucleii, while the other slower-acting acids release gas during bench action or baking.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days