Trimethylsilyl trifluoromethanesulfonate CAS#27607-77-8
CAS Number: 27607-77-8
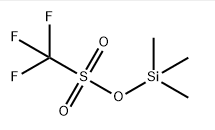
Chemical Formula: C4H9F3O3SSi
Synonyms:
TRIMETHYLSILYL TRIFLUOROMETHANESULPHONATE
CT3795
Methanesulfonic acid, trifluoro-, trimethylsilyl ester
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Appearance: White Powder
Trimethylsilyl trifluoromethanesulfonate CAS#27607-77-8
Trimethylsilyl Trifluoromethane sulfonate is generally used following reactions:
1. Silylation. TMSOTf is widely used in the conversion of car bonyl compounds to their enol ethers. The conversion is some 109 faster with TMSOTf/triethylamine than with chlorotrimethy lsilane.Dicarbonyl compounds are converted to the corresponding bis enol ethers; this method is an improvement over the previous two step method.In general, TMSOTf has a tendency toC-silylation which is seen most clearly in the reaction of esters, whereC-silylation dominates over O-silylation.
2.Carbonyl Activation. 1,3-Dioxolanation of conjugated enals is facilitated by TMSOTf in the presence of 1,2-bis(trimethylsilyl oxy)ethane. In particular, highly selective protection of sterically differentiated ketones is possible (eq 10).TMSOTf mediates a stereoselective aldol-type condensation of silyl enol ethers and acetals (or orthoesters). The nonbasic reaction conditions are extremely mild. The use of TMSOTf in aldol reactions of silyl enol ethers and ketene acetals with aldehydes is ubiquitous. Stereoselective cyclization of α,β-unsaturated enamide esters is induced by TMSOTf and has been used as a route to quinolizidines and indolizidines.
Trimethylsilyl trifluoromethanesulfonate Chemical Properties |
Melting point | 25°C |
Boiling point | 77 °C/80 mmHg (lit.) |
density | 1.228 g/mL at 25 °C (lit.) |
vapor pressure | 4.7 hPa (20 °C) |
refractive index | n |
Fp | 78 °F |
storage temp. | Store below +30°C. |
solubility | sol aliphatic and aromatic hydrocarbons, haloalkanes, ethers. |
form | Fuming Liquid |
color | Clear colorless to light brown |
Specific Gravity | 1.15 |
Water Solubility | REACTS |
Sensitive | Moisture Sensitive |
Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
Merck | 14,9719 |
BRN | 1868911 |
InChIKey | FTVLMFQEYACZNP-UHFFFAOYSA-N |
CAS DataBase Reference | 27607-77-8(CAS DataBase Reference) |
NIST Chemistry Reference | Trimethylsilyl trifluoromethanesulfonate(27607-77-8) |
EPA Substance Registry System | Methanesulfonic acid, trifluoro-, trimethylsilyl ester (27607-77-8) |
Safety Information |
Hazard Codes | C,F |
Risk Statements | 10-14-34 |
Safety Statements | 16-26-36/37/39-45-8 |
RIDADR | UN 2920 8/PG 2 |
WGK Germany | 3 |
F | 10-21 |
Autoignition Temperature | 405 °C DIN 51794 |
Hazard Note | Corrosive/Flammable |
TSCA | TSCA listed |
HazardClass | 3 |
PackingGroup | III |
HS Code | 29310095 |
Product Application of Trimethylsilyl trifluoromethanesulfonate CAS#27607-77-8
Trimethylsilyl Trifluoromethanesulfonate is a trialkylsilyl triflate used as a catalyst in organic synthesis. Trimethylsilyl Trifluoromethanesulfonate is used in combination with boron trifluoride eth yl ether to prepare a Lewis acid that is more powerful than its components and especially effective in acetonitrile solvent. Trimethylsilyl is a common reagent used in a Dieckmann-like cyclization of ester-imides and diesters.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days