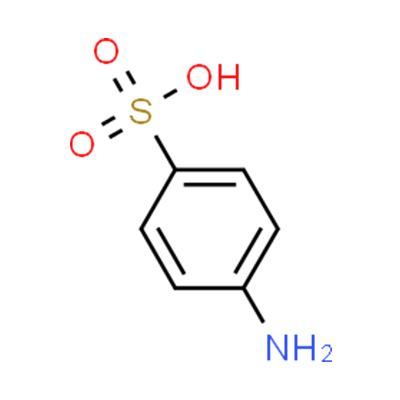
Sulfanilic acid CAS#121-57-3
CAS Number: 121-57-3
Chemical Formula: C6H7NO3S
Synonyms:
4-aminobenzenesulfonate
Diazobenzenesulfonic acid test solution(ChP)
p-Anilinesulfonic acid anhydrous
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Appearance: White Powder
Sulfanilic acid CAS#121-57-3
Sulfanilic acid (4-amino benzene sulfonic acid ) is an off-white crystalline solid which finds application in quantitative analysis of nitrate and nitrite ions. The solid acid exists as a zwit
Sulfanilic acid Chemical Properties |
Melting point | >300 °C(lit.) |
Boiling point | 288 ℃ |
bulk density | 620kg/m3 |
density | 1.485 |
vapor pressure | 0Pa at 25℃ |
refractive index | 1.5500 (estimate) |
storage temp. | Store below +30°C. |
solubility | 10g/l |
pka | 3.24(at 25℃) |
form | solid |
color | White to Off-White |
PH | 2.5 (10g/l, H2O, 20℃) |
Water Solubility | 0.1 g/100 mL (20 ºC) |
Merck | 14,8926 |
BRN | 908765 |
Stability: | Stable. Incompatible with strong oxidizing agents. |
InChI | 1S/C6H7NO3S/c7-5-1-3-6(4-2-5)11(8,9)10/h1-4H,7H2,(H,8,9,10) |
InChIKey | HVBSAKJJOYLTQU-UHFFFAOYSA-N |
SMILES | Nc1ccc(cc1)S(O)(=O)=O |
LogP | -2.3 at 25℃ |
CAS DataBase Reference | 121-57-3(CAS DataBase Reference) |
NIST Chemistry Reference | Benzenesulfonic acid, 4-amino-(121-57-3) |
EPA Substance Registry System | Sulfanilic acid (121-57-3) |
Safety Information |
Hazard Codes | C,Xi |
Risk Statements | 36/38-43-34 |
Safety Statements | 26-36/37/39-45-37-24-36/37 |
RIDADR | UN 2790 8/PG 3 |
WGK Germany | 1 |
RTECS | WP3895500 |
Autoignition Temperature | >400 °C |
TSCA | TSCA listed |
HS Code | 29214210 |
Storage Class | 11 - Combustible Solids |
Hazard Classifications | Eye Irrit. 2 |
Hazardous Substances Data | 121-57-3(Hazardous Substances Data) |
Toxicity | LD50 orally in Rabbit: 12300 mg/kg |
Product Application Of Sulfanilic acid CAS#121-57-3
As the compound readily form diazo compounds, it is used to make dyes and sulpha drugs . This property is also used for the quantitative analysis of nitrate and nitrite ions by diazonium coupling reaction with N-(1-Naphthyl) ethylene diamine , resulting in an azo dye, and the concentration of nitrate or nitrite ions were deduced from the color intensity of the resulting red solution by colorimetry. |
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days