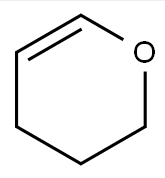
3,4-Dihydro-2H-pyran CAS# 110-87-2
CAS Number: 110-87-2
Chemical Formula: C5H8O
Synonyms:
DIHYDROPYRAN
δ2-dihydropyran
delta(Sup2)-Dihydropyran
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Appearance: Transperant liquid
3,4-Dihydro-2H-pyran CAS# 110-87-2
Widely used hydroxyl-protecting reagent3,4-Dihydro-2H-pyran is used as a hydroxyl-protecting reagent in organic synthesis. It acts as an intermediate in synthetic chemistry. It is used to protect various reactive functional groups. It is involved in the polymerization reaction either alone or with unsaturated compound and finds application in polymer industries. Further, it is employed in the preparation of bicyclic compounds of epoxide-fused, halo compounds and allenic alcohols.
3,4-Dihydro-2H-pyran Chemical Properties |
Melting point | -70 °C (lit.) |
Boiling point | 86 °C (lit.) |
density | 0.922 g/mL at 25 °C (lit.) |
vapor density | 2.9 (vs air) |
vapor pressure | 77.8 hPa (20 °C) |
refractive index | n |
Fp | 4 °F |
storage temp. | Store below +30°C. |
solubility | 7.7g/l |
form | Liquid |
color | Clear colorless to yellow |
PH | 7 (5g/l, H2O, 20℃) |
explosive limit | 1.1-13.8%(V) |
Water Solubility | 20 g/L (20 ºC) |
BRN | 103493 |
Stability: | Volatile |
InChIKey | BUDQDWGNQVEFAC-UHFFFAOYSA-N |
LogP | 0.690 |
CAS DataBase Reference | 110-87-2(CAS DataBase Reference) |
NIST Chemistry Reference | 2H-Pyran, 3,4-dihydro-(110-87-2) |
EPA Substance Registry System | 2H-Pyran, 3,4-dihydro- (110-87-2) |
Safety Information |
Hazard Codes | F,Xi |
Risk Statements | 11-36/37/38-36/38-19 |
Safety Statements | 16-26-36-37/39-33-7/9 |
RIDADR | UN 2376 3/PG 2 |
WGK Germany | 3 |
RTECS | UP7700000 |
Autoignition Temperature | 240 °C |
Hazard Note | Highly Flammable/Irritant |
TSCA | Yes |
HazardClass | 3 |
PackingGroup | II |
HS Code | 29329995 |
Toxicity | LD50 orally in Rabbit: > 4000 mg/kg |
Product Application of 3,4-Dihydro-2H-pyran CAS# 110-87-2
3,4-Dihydro-2H-pyran can be used as a reactant to synthesize:
Tetrahydropyranylated product from alcohols in the presence of phenolsulfonic acid-formaldehyde resin catalyst.
Tetrahydropyran derivatives by reacting pyrazoles in the presence of trifluoroacetic acid.
1,2,3,4-Tetrahydroquinoline derivatives by cation-exchange resin catalyzed Domino reaction with aromatic amines.
Tetrahydroquinolines from aryl azides in the presence of FeCl3-NaI.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days