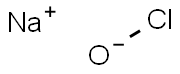Sodium Hypochlorite CAS#7681-52-9
CAS Number: 7681-52-9
Chemical Formula: ClNaO
Synonyms:
epapesticidechemicalcode014703
hypochloritesolutioncontaining>7%avaliablechlorinebywt.(un1791)
Hypochlorousacid,sodiumsalt
Appearance: Light yellow Solution
HS Code: 28289000
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Products Description of Sodium hypochlorite CAS#7681-52-9
Sodium hypochlorite, NaOCl, is an air-unstable,pale inexperienced crystalline stable that is soluble in bloodless water, decomposes in warm water, and has a candy aroma. It commonly is on hand in one of two strengths. The family liquid bleach consists of about 5.25 wt% NaCIO. The business product(sometimes referred to as 15% bleach) consists of 150g/L handy chlorine.
Parameters
Melting point | -16 °C |
Boiling point | 111 °C |
density | 1.25 g/mL at 20 °C |
vapor pressure | 17.5 mmHg ( 20 °C) |
refractive index | 1.3870 |
storage temp. | 2-8°C |
solubility | Methanol (Slightly), Water (Slightly) |
form | Solution |
color | Light yellow |
Specific Gravity | 1.209 |
Odor | pale greenish to yel. liq., chlorine bleach odor |
PH | 12-13 (20°C, 68 °F) |
Water Solubility | decomposes. |
Merck | 14,8628 |
Dielectric constant | 6.7(Ambient) |
Exposure limits | ACGIH: Ceiling 2 mg/m3 |
BCS Class | 1 |
Stability: | Stable. Contact with acids releases poisonous gas ( chlorine ). Light sensitive. Incompatible with strong acids, amines, ammonia, ammonium salts, reducing agents, metals, aziridine, methanol, formic acid, phenylacetonitrile. |
LogP | -3.42 at 20℃ |
CAS DataBase Reference | 7681-52-9(CAS DataBase Reference) |
EPA Substance Registry System | Sodium hypochlorite (7681-52-9) |
Safety Information
Hazard Codes | C,Xi,N |
Risk Statements | 31-34-36/38-36/37/38-50 |
Safety Statements | 26-36/37/39-45-50A-28A-36-61-50-28 |
RIDADR | UN 1791 8/PG 3 |
WGK Germany | 2 |
RTECS | NH3486300 |
TSCA | Yes |
HazardClass | 8 |
PackingGroup | III |
HS Code | 28289000 |
Hazardous Substances Data | 7681-52-9(Hazardous Substances Data) |
Toxicity | Skin contact with the solid hypochlorite pentahydrate or its concentrated solution can cause irritation. Ingestion may cause corrosion of mucous membranes and gastric perforation. |
Product Application of Sodium hypochlorite CAS#7681-52-9
Sodium hypochlorite is marketed solely as an aqueous answer due to the fact the anhydrous strong is noticeably unstable and can explode. The stable pentahydrate additionally is unstable in air, decomposed by way of response with carbon dioxide from air. Aqueous options are very stable. They are used for bleaching textiles and paper pulp; in cleansing solutions; in water purification; as a disinfectant for swimming pools; and as a germicide and topical antiinfective.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days