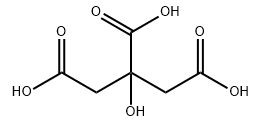
Citric acid CAS#77-92-9
CAS Number: 77-92-9
Chemical Formula: C6H8O7
Synonyms:
CITRIC ACID FREE ACID, ANHYDROUS PLANTCE LL CULTURE
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Appearance: White Powder
Citric acid is a white, crystalline, weak organic acid present in most plants and many animals as an intermediate in cellular respiration. Citric acid contains three carboxyl groups making it a carboxylic, more specifically a tricarboxylic, acid.the name citrus originates from the Greek kedromelon meaning apple of melon for the fruit citron. Greek works mention kitron, kitrion, or kitreos for citron fruit, which is an oblong fruit several inches long from the scrublike tree Citrus medica. Lemons and limes have high citric acid content, which may account for up to 8% of the fruit's dry weight.
| Citric acid Chemical Properties |
| Melting point | 153-159 °C (lit.) |
| Boiling point | 310 °C (decomp) |
| bulk density | 560kg/m3 |
| density | 1.67 g/cm3 at 20 °C |
| vapor density | 7.26 (vs air) |
| vapor pressure | <0.1 hPa (20 °C) |
| refractive index | 1.493~1.509 |
| FEMA | 2306 | CITRIC ACID |
| Fp | 100 °C |
| storage temp. | 2-8°C |
| solubility | Citric acid also dissolves in absolute (anhydrous) ethanol (76 parts of citric acid per 100 parts of ethanol) at 15 °C. |
| form | grit |
| pka | 3.14(at 20℃) |
| color | White |
| PH | 3.24(1 mM solution);2.62(10 mM solution);2.08(100 mM solution); |
| Odor | Odorless |
| Odor Type | odorless |
| biological source | synthetic |
| explosive limit | 8%, 65°F |
| Water Solubility | soluble in Water (1174g/L at 10°C, 1809g/L at 30°C, 3825g/L at 80°C). |
| Sensitive | Hygroscopic |
| λmax | λ: 260 nm Amax: 0.20 λ: 280 nm Amax: 0.10 |
| Merck | 14,2326 |
| JECFA Number | 218 |
| BRN | 782061 |
| Stability: | Stable. Incompatible with bases, strong oxidizing agents, reducing agents, metal nitrates. |
| Cosmetics Ingredients Functions | CHELATING FRAGRANCE BUFFERING |
| InChIKey | KRKNYBCHXYNGOX-UHFFFAOYSA-N |
| LogP | -1.64 |
| CAS DataBase Reference | 77-92-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,2,3-Propanetricarboxylic acid, 2-hydroxy-(77-92-9) |
| EPA Substance Registry System | Citric acid (77-92-9) |
| Safety Information |
| Hazard Codes | Xi,C,T |
| Risk Statements | 41-36/37/38-36/38-37/38-34-36-35-61-60 |
| Safety Statements | 26-39-37/39-24/25-36/37/39-45-36-53 |
| RIDADR | UN 1789 8/PG 3 |
| WGK Germany | 1 |
| RTECS | GE7350000 |
| F | 9 |
| TSCA | TSCA listed |
| HS Code | 2918 14 00 |
| Hazardous Substances Data | 77-92-9(Hazardous Substances Data) |
| Toxicity | LD50 in mice, rats (mmol/kg): 5.0, 4.6 i.p. (Gruber, Halbeisen) |
Product Application of Citric acid CAS#77-92-9
| Citric acid is a weak organic acid that is known as a commodity chemical, as more than a million tonnes are produced every year by mycological fermentation on an industrial scale using crude sugar sol utions, such as molasses and strains of Aspergillus niger. Citric acid is widely distributed in plants and in animal tissues and fluids and exist in greater than grace amounts in variety of fruits and vegetables, most notably in citrus fruits such as lemon and limes. Citric acid is mainly used as an acidifier, flavoring agent and chelating agent. It was also used as a chemical restrainer particularly in developers for the collodion process and in silver nitrate solutions used for sensitizing salted and albumen papers. |
Strontium carbonate CAS#1633-05-2
Strontium carbonate (SrCO3)(1633-05-2) belongs to the carbonate salt of strontium, which is found in nature as the mineral strontianite. It can be applied in a variety of industries. At present, strontium carbonates are commonly being applied as an inexpensive colorant in pyrotechnics since strontium and its salts produce a crimson read flame. Strontium carbonate, in general, is preferred in fireworks, compared with other strontium salts due to its inexpensive cost, nonhygroscopic property, and ability to neutralize acid. It can also be used as road flares and for preparing iridescent glass, luminous paints, strontium oxide or strontium salts and in refining sugar and certain drugs. It is also recommended as a substitute for barium to produce matte glazes. Besides, its applications involves in ceramics industry, where it serves as an ingredient in glazes, and in electric products, where it is used for the production of strontium ferrites to produce permanent magnets for loudspeakers and door magnets. Strontium carbonate is also used for manufacturing some superconductors such as BSCCO and also for electroluminescent materials.
| Strontium carbonate Chemical Properties |
| Melting point | 1494 °C (lit.) |
| density | 3.7 g/mL at 25 °C (lit.) |
| refractive index | 1.518 |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | dilute aqueous acid: slightly soluble(lit.) |
| form | Powder |
| Specific Gravity | 3.7 |
| color | white |
| PH | 7-8 (20°C, 0.01g/L in H2O) |
| Odor | Odorless |
| Water Solubility | Soluble in ammonium chloride. Slightly soluble in ammonia and water. |
| Merck | 14,8838 |
| Solubility Product Constant (Ksp) | pKsp: 9.25 |
| Stability: | Stable. Incompatible with strong acids. |
| InChIKey | LEDMRZGFZIAGGB-UHFFFAOYSA-L |
| LogP | -0.809 (est) |
| CAS DataBase Reference | 1633-05-2(CAS DataBase Reference) |
| EPA Substance Registry System | Carbonic acid, strontium salt (1:1) (1633-05-2) |
| Safety Information |
| WGK Germany | - |
| TSCA | TSCA listed |
| HS Code | 2836920000 |
| Hazardous Substances Data | 1633-05-2(Hazardous Substances Data) |
Product Application of Strontium carbonate CAS#1633-05-2
The compound, SrCO3, is used in pyrotechnics and ceramic ferrites. It is also used in making iridescent glass for color television tubes. Other uses are in refining sugar and preparing other strontium salts. The most common use is as an inexpensive fireworks colorant. Strontium and its salts emit a brilliant red color in flame. Its ability to neutralize acid is also very helpful in pyrotechnics. Another similar application is in road flares. Strontium carbonate is used for electronic applications. It is used for manufacturing glass colortelevision tubes to absorb X-rays resulting from the bombardment of the cathode rays on the glass enclosure of the cathode-ray gun. SrCO3 is used in the preparation of iridescent glass, strontium oxide or strontium salts and in refining sugar.It is widely used in the ceramics industry as an ingredient in glazes. It acts as a flux and also modifies the color of certain metallic oxides. It is also used in the manufacturing of strontium ferrites for permanent magnets that are used in loudspeakers and door-magnets. Strontium carbonate can be used to produce many different strontium compounds by simply dissolving it in the corresponding acid. Strontium bicarbonate has not been isolated.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days