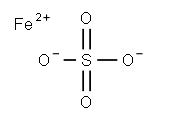Ferrous sulfate CAS#7720-78-7
CAS Number: 7720-78-7
Chemical Formula: FeO4S
Synonyms:
Iron(Ⅱ) sulfate
ferric potassium alum
COPPERAS
MOQ (Minimum Order Quantity): 1 FCL (Full Container Load)
Appearance: White Powder
Ferrous sulfate CAS#7720-78-7
Green vitriol (FeSO₄·7H₂O) has been known since the thirteenth century; it crystallizes from solutions of iron or iron bases in dilute sulfuric acid. The heptahydrate forms green monoclinic crystals with a density of 1.88 and is very soluble in water (296 g L⁻¹ FeSO₄ at 25 °C). The white monohydrate is obtained by precipitating the aqueous solution with ethanol, heating the heptahydrate to 140 °C in vacuo, or crystallizing it from 50% sulfuric acid. This can be further dehydrated to white, amorphous FeSO₄ by heating to 300 °C in a stream of hydrogen. At red heat, the sulfate decomposes:
2FeSO₄ → Fe₂O₃ + SO₂ + SO₃ A tetrahydrate (FeSO₄·4H₂O) crystallizes from aqueous solutions above 56 °C.
A rusty-brown solid prepared by the action of heat on iron(III) hydroxide or iron(II) sulfate. It occurs in nature as the mineral hematite. Industrially it is obtained by roasting iron pyrites. Iron(III) oxide dissolves in dilute acids to produce solutions of iron(III) salts. It is stable at red heat, decomposes around 1300°C to give triiron tetroxide, and can be reduced to iron by hydrogen at 1000°C. Iron(III) oxide is not ionic in character but has a structure similar to that of aluminum(III) oxide.
Ferrous sulfate Chemical Properties |
Melting point | decomposes at 671℃ [JAN85] |
density | 3.650 |
vapor pressure | 0Pa at 20℃ |
storage temp. | Store at +15°C to +25°C. |
solubility | Water (Slightly) |
form | white orthorhombic crystals |
color | white orthorhombic crystals, crystalline; hygroscopic |
Odor | at 100.00?%. odorless |
PH | 2.5 (20°C, 50g/L in H2O) |
Water Solubility | g/100g solution H2O: 13.6 (0°C), 22.8 (25°C), 24.0 (100°C); solid phase, FeSO4 · 7H2O (0°C, 25°C), FeSO4 ·H2O (100°C) [KRU93] |
Dielectric constant | 14.2(14℃) |
Cosmetics Ingredients Functions | ASTRINGENT |
InChI | InChI=1S/Fe.H2O4S/c;1-5(2,3)4/h;(H2,1,2,3,4)/q+2;/p-2 |
InChIKey | BAUYGSIQEAFULO-UHFFFAOYSA-L |
SMILES | [Fe+2].S([O-])([O-])(=O)=O |
CAS DataBase Reference | 7720-78-7(CAS DataBase Reference) |
NIST Chemistry Reference | Ferrous sulfate(7720-78-7) |
EPA Substance Registry System | Ferrous sulfate (7720-78-7) |
Safety Information |
Risk Statements | 25 |
Safety Statements | 45 |
HS Code | 28332910 |
Hazardous Substances Data | 7720-78-7(Hazardous Substances Data) |
Toxicity | LD50 oral in rat: 319mg/kg |
Product Application of Ferrous sulfate CAS#7720-78-7
Ferrous Sulfate is a nutrient and dietary supplement that is a source of iron. it is a white to grayish odorless powder. ferrous sulfate hep- tahydrate contains approximately 20% iron, while ferrous sulfate dried contains approximately 32% iron. it dissolves slowly in water and has high bioavailability. it can cause discoloration and rancidity. it is used for fortification of baking mixes. in the encapsulated form it does not react with lipids in cereal flours. it is used in infant foods, cereals, and pasta products.
Ferrous sulfate (FeSO4) is also known as iron sulfate or iron vitriol. It is used in the production of various chemicals, such as sulfur dioxide and sulfuric acid.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days