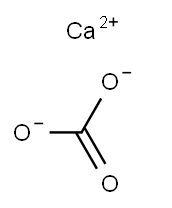
Calcium carbonate CAS#471-34-1
Calcium carbonate occurs in nature as limestone in various forms, such as marble, chalk, and coral. It is probably the most widely-used raw material in the chemical industry. It has numerous applications, primarily to produce cement, mortars, plasters, refractories, and glass as building materials. It also is used to produce quicklime, hydrated lime and a number of calcium compounds. It is produced either as powdered or precipitated calcium carbonate. The latter consists of finer particles of greater purity and more uniform size. They also have many important commercial applications. Various grades of precipitated calcium carbonate are used in several products, such as textiles, papers, paints, plastics, adhesives, sealants, and cosmetics.
| Calcium carbonate Chemical Properties |
| Melting point | 825 °C |
| Boiling point | 800 °C |
| bulk density | 300-1400kg/m3 |
| density | 2.93 g/mL at 25 °C (lit.) |
| refractive index | 1.6583 |
| storage temp. | Store at +5°C to +30°C. |
| solubility | 5 M HCl: 0.1 M at 20 °C, clear, colorless |
| form | random crystals |
| color | White-beige to slightly beige-gray |
| Specific Gravity | 2.93 |
| Odor | Odorless |
| PH | 9.91(1 mM solution);9.91(10 mM solution);9.91(100 mM solution); |
| PH Range | 8 |
| Flame Color | Red-orange |
| Water Solubility | Insoluble |
| λmax | λ: 260 nm Amax: ≤0.09 λ: 280 nm Amax: ≤0.06 |
| Merck | 14,1657 |
| Solubility Product Constant (Ksp) | pKsp: 8.54 |
| BRN | 8008338 |
| Dielectric constant | 6.1(Ambient) |
| Exposure limits | NIOSH: TWA 10 mg/m3; TWA 5 mg/m3 |
| Stability: | Stable. Incompatible with acids, fluorine, ammonium salts, alum. |
| Cosmetics Ingredients Functions | BULKING BUFFERING ABRASIVE ORAL CARE COLORANT OPACIFYING |
| InChIKey | VTYYLEPIZMXCLO-UHFFFAOYSA-L |
| Modulus of Elasticity | 10.0 - 80.0 GPa |
| Hardness, Knoop | 75 - 120 |
| Hardness, Mohs | 2.0 - 5.0 |
| Hardness, Shore H | 10 - 60 |
| Hardness, Vickers | 105 - 136 |
| Drilling Hardness | 50 |
| CAS DataBase Reference | 471-34-1(CAS DataBase Reference) |
| EPA Substance Registry System | Calcium carbonate (471-34-1) |
| Safety Information |
| Hazard Codes | Xi |
| Risk Statements | 37/38-41-36/38-36 |
| Safety Statements | 26-36/37/39-37/39-37 |
| OEB | B |
| OEL | TWA: 10 mg/m3 (total) |
| WGK Germany | - |
| RTECS | FF9335000 |
| TSCA | Yes |
| HS Code | 28365000 |
| Hazardous Substances Data | 471-34-1(Hazardous Substances Data) |
| Toxicity | LD50 orally in Rabbit: 6450 mg/kg LD50 dermal Rat > 2000 mg/kg |
Product Application of Calcium carbonate CAS#471-34-1
Calcium Carbonate is the calcium salt of carbonic acid which is used as an anticaking agent and dough strengthener. it is available in varying particle sizes ranging from coarse to fine powder. it is practically insoluble in water and alcohol, but the presence of any ammonium salt or carbon dioxide increases its solubility while the presence of any alkali hydroxide reduces its solubility. it has a ph of 9–9.5. it is the primary source of lime (calcium oxide) which is made by heating limestone in a furnace. calcium carbonate is used as a filler in baking powder, for calcium enrichment, as a mild buffering agent in doughs, as a source of calcium ions in dry mix desserts, and as a neutralizer in antacids. it is also termed limestone.
Factory and Equipment Show
Fast delivery time
Inventory 2-3 working days New production 7-10 working days